Exploring the Periodic Table at West Carter
I had a great time with some West Carter Middle school students showing them a little about the chemical elements.
We investigated density with a lead brick and another brick the same size but with a much lower density for comparison.
The lead weighed thirteen pounds but seemed much heaver to them.
I told them to try to grab both and pick them up evenly. Not easy.





We passed around blocks that all weighed the same but were made of a variety of materials.
They were made of: lead, iron, copper, brass, aluminum, graphite, lignum vite wood, lithium (simulated), oak, pine and balsa.
Some insisted that the smaller
samples "had to" weigh more than the big ones. It is interesting
how your senses shouldn't be trusted at times.


A block of magnesium was another density surprise.

On to a couple illustrations of chemical change that were also unexpected.
We rapidly oxidized (that is burned) some metals.
First iron (in the form of steel wool) then magnesium.


All of you who are looking at this
page are taking advantage of the special electrical properties of the
element shown here (silicon).
It is a semiconductor that can be used to control the flow of electrons that are carrying signals that you are using.

Computer chips are made of it and
their electrical properties depend on tiny amounts of specific
elemental impurities that are added during their manufacture.
Elements that may be used to make the transistors on the chip include: boron, phosphorous, antimony, and arsenic.
Aluminum or copper are used to
connect the transistors together on this chip and fine gold wires
connect from this chip to the pins that carry signals and power.




When I asked what is your favorite element this one (gold) was the second most common answer.
Only oxygen ranked higher.


A lump of very pure germanium that
was once part of a radiation detector gave them a chance to see another
semiconducting element.



You can sort out nickel, iron and cobalt samples.
They are the only elements that stick to a magnet.


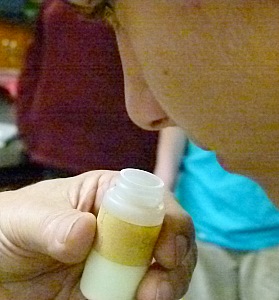
I don't recommend sniffing samples to determine what they are but in this case it was OK.
Sulfur is known to be of low toxicity, and poses very little if any risk
to human health.
It is easily recognized by color and smell once you have been introduced to it.
The sample I am holding is sulfur as it is found near volcanic vents.


This collection of elements let the kids see what each of them looked like in it's pure form.
Platinum, silver, gold, nickel, copper, and iron all look like you probably expect.
Gray pellets of calcium seemed to be the most unexpected. You thought it would be white didn't you.
They were also fascinated by the two
elements that are liquid at room temperature and the one that melts at
body temperature: bromine, mercury, and gallium.







On to another way to distinguish the elements, their spectra.
Here we have flames showing the color characteristic of 5 different elements.
Actually because of over and underexposure of the various flames the photo is a poor representation of the colors.
In person your eye is much better at recognizing the differences.

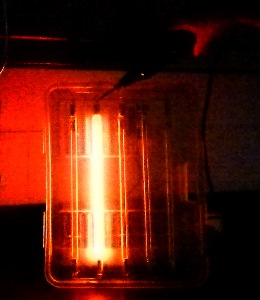
We looked at the differences in the color of an electrical discharge in each of the noble gases (except for radioactive radon).
The bright orange one is neon. The others are argon and xenon.
By looking through diffraction gratings they could see the spectral lines that were characteristic of each.
We also talked about the discovery of helium and xenon on the sun before they were known on earth.







At the beginning of each class I asked if we had any photographers in the room then handed my camera to one of those students.
The pictures above are the result.
I like what we got. Even the last set which had very little light turned out well. My thanks to the photographers.
We discussed the discovery of the periodic table and I took them on a virtual visit with Mendeleev and a tour of his lab.

They also enjoyed the Tom Lehrer elements song. Two versions of which you can hear here and here.
Here are a couple of related web sites you might enjoy visiting:
Theo Gray's periodic table with pictures of each of the elements in his collection.
The University of Nottingham has videos about each of the elements.
I hope that you enjoyed your visit to
this page. If you want to explore more with us you could visit
one of the links below.
Go to our Science Fun
page
Go to our Travels page
Go to our Personal home
page
Go to our Community page
E-mail Nancy and Alan

www.mrtc.com/anvk web site by Alan Kuehner is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License.
Permissions beyond the scope of this license may be available at http://members.mrtc.com/anvk/permit.html.

